when an atom bonds to another atom what is it trying to do
What you'll learn to do: Classify dissimilar types of atomic bonds
When atoms bail together, they create molecules: a sodium atom bonds with a chlorine atom to create table salt (sodium chloride), two hydrogen atoms bond with an oxygen atom to create h2o (hydrogen dioxide). However, not all atomic bonds are the same; in fact salt and water are created with 2 very different types of bonds (ionic and polar covalent bonds respectively).
The dissimilar types of bonds (ionic, polar covalent, and non-polar covalent bonds) behave differently, and these differences accept an impact on the molecules they create. Agreement the types of bonds that create living things tin help us sympathise those living things themselves.
Learning Outcomes
- Define the octet rule and its role in chemic bonds
- Describe the characteristics of ionic bonds and place common ions
- Describe the characteristics of covalent bonds and differentiate between polar and nonpolar bonds
- Model a Hydrogen bond and identify its unique qualities
- Model van der Waals interactions place their unique qualities
- Describe the properties of water that are critical to maintaining life
Chemic Bonding
Not all elements have enough electrons to make full their outermost shells, but an cantlet is at its about stable when all of the electron positions in the outermost shell are filled. Because of these vacancies in the outermost shells, nosotros see the formation of chemical bonds, or interactions between 2 or more of the same or unlike elements that result in the formation of molecules. To achieve greater stability, atoms will tend to completely make full their outer shells and will bond with other elements to accomplish this goal by sharing electrons, accepting electrons from another atom, or altruistic electrons to another cantlet. Because the outermost shells of the elements with low atomic numbers (upward to calcium, with atomic number 20) can hold eight electrons, this is referred to as the octet rule. An element tin donate, accept, or share electrons with other elements to fill its outer shell and satisfy the octet rule.
An early model of the atom was developed in 1913 by the Danish scientist Niels Bohr (1885–1962). The Bohr model shows the cantlet as a central nucleus containing protons and neutrons, with the electrons in circular electron shells at specific distances from the nucleus, similar to planets orbiting around the sun. Each electron shell has a different energy level, with those shells closest to the nucleus being lower in energy than those farther from the nucleus. Past convention, each beat out is assigned a number and the symbol north—for example, the electron shell closest to the nucleus is called 1n. In order to move between shells, an electron must blot or release an corporeality of energy corresponding exactly to the difference in energy between the shells. For instance, if an electron absorbs free energy from a photon, information technology may become excited and motility to a higher-energy shell; conversely, when an excited electron drops back down to a lower-energy shell, it will release free energy, ofttimes in the form of heat.
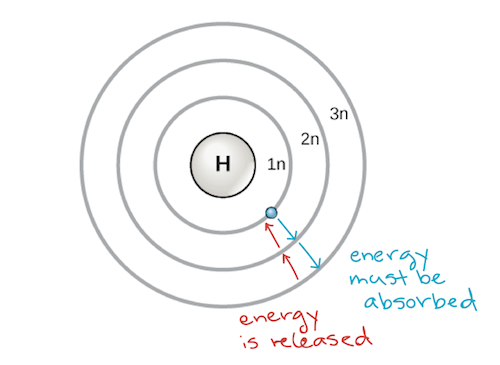
Bohr model of an atom, showing energy levels as concentric circles surrounding the nucleus. Energy must exist added to move an electron outward to a higher energy level, and energy is released when an electron falls downwards from a higher free energy level to a closer-in one. Prototype credit: modified from OpenStax Biological science
Atoms, similar other things governed by the laws of physics, tend to take on the lowest-free energy, most stable configuration they can. Thus, the electron shells of an atom are populated from the within out, with electrons filling up the low-free energy shells closer to the nucleus before they move into the higher-free energy shells further out. The shell closest to the nucleus, 1n, can concur two electrons, while the adjacent shell, 2n, can hold eight, and the 3rd beat, 3n, can concord up to eighteen.
The number of electrons in the outermost crush of a item cantlet determines its reactivity, or tendency to class chemical bonds with other atoms. This outermost vanquish is known as thevalence trounce, and the electrons establish in it are calledvalence electrons. In full general, atoms are almost stable, least reactive, when their outermost electron shell is full. Most of the elements of import in biological science demand 8 electrons in their outermost shell in club to exist stable, and this rule of thumb is known as theoctet dominion. Some atoms tin be stable with an octet even though their valence trounce is the 3n shell, which tin hold upward to xviii electrons. We will explore the reason for this when we discuss electron orbitals below.
Examples of some neutral atoms and their electron configurations are shown beneath. In this table, y'all tin can see that helium has a full valence shell, with ii electrons in its showtime and simply, 1n, shell. Similarly, neon has a complete outer 2n shell containing viii electrons. These electron configurations make helium and neon very stable. Although argon does not technically accept a total outer shell, since the 3n shell can agree up to eighteen electrons, it is stable like neon and helium because information technology has eight electrons in the 3n shell and thus satisfies the octet dominion. In contrast, chlorine has simply 7 electrons in its outermost trounce, while sodium has just one. These patterns do non fill the outermost shell or satisfy the octet dominion, making chlorine and sodium reactive, eager to gain or lose electrons to reach a more stable configuration.
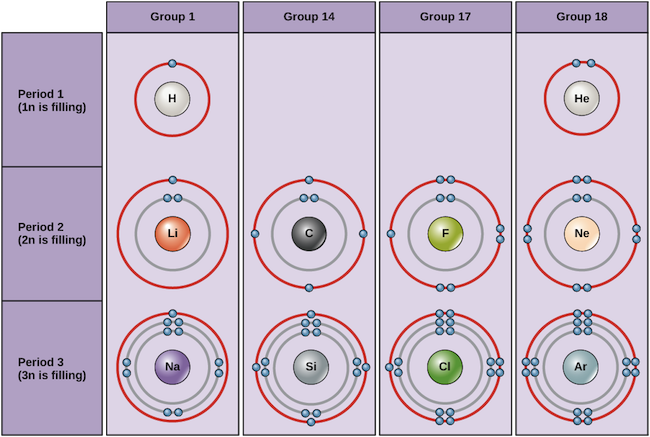
Bohr diagrams of various elements Paradigm credit: OpenStax Biology
Electron configurations and the periodic table
Elements are placed in order on the periodic table based on their atomic number, how many protons they have. In a neutral atom, the number of electrons will equal the number of protons, and then we can easily determine electron number from atomic number. In addition, the position of an element in the periodic table—its column, or group, and row, or flow—provides useful information almost how those electrons are arranged.
If we consider just the first three rows of the table, which include the major elements of import to life, each row corresponds to the filling of a unlike electron beat out: helium and hydrogen place their electrons in the 1n crush, while second-row elements like Li start filling the 2n crush, and third-row elements similar Na continue with the 3n shell. Similarly, an element'southward column number gives information about its number of valence electrons and reactivity. In full general, the number of valence electrons is the same within a column and increases from left to right within a row. Group 1 elements accept only one valence electron and grouping eighteen elements have viii, except for helium, which has only 2 electrons total. Thus, group number is a good predictor of how reactive each chemical element will be:
- Helium (He), neon (Ne), and argon (Ar), as group 18 elements, accept outer electron shells that are full or satisfy the octet rule. This makes them highly stable equally single atoms. Considering of their non-reactivity, they are called theinert gases ornoble gases.
- Hydrogen (H), lithium (Li), and sodium (Na), equally group 1 elements, have just i electron in their outermost shells. They are unstable as unmarried atoms, simply tin can get stable by losing or sharing their one valence electron. If these elements fully lose an electron—as Li and Na typically do—they get positively charged ions: Li+, Na+.
- Fluorine (F) and chlorine (Cl), as grouping 17 elements, take vii electrons in their outermost shells. They tend to reach a stable octet by taking an electron from other atoms, becoming negatively charged ions: F− and Cl−.
- Carbon (C), every bit a group fourteen chemical element, has iv electrons in its outer shell. Carbon typically shares electrons to reach a complete valence shell, forming bonds with multiple other atoms.
Thus, the columns of the periodic tabular array reflect the number of electrons found in each element's valence shell, which in plow determines how the element will react.
Ionic Bonds
Some atoms are more stable when they gain or lose an electron (or perchance two) and form ions. This fills their outermost electron shell and makes them energetically more stable. Because the number of electrons does not equal the number of protons, each ion has a net charge.Cations are positive ions that are formed past losing electrons. Negative ions are formed past gaining electrons and are called anions. Anions are designated by their elemental name being altered to end in "-ide": the anion of chlorine is called chloride, and the anion of sulfur is called sulfide, for example.
This movement of electrons from one element to another is referred to aselectron transfer. As Figure 1 illustrates, sodium (Na) merely has ane electron in its outer electron shell. It takes less energy for sodium to donate that one electron than it does to accept seven more electrons to fill up the outer shell. If sodium loses an electron, it now has 11 protons, 11 neutrons, and only 10 electrons, leaving it with an overall charge of +1. It is now referred to as a sodium ion. Chlorine (Cl) in its lowest energy land (chosen the ground state) has vii electrons in its outer shell. Again, it is more than energy-efficient for chlorine to gain one electron than to lose 7. Therefore, it tends to proceeds an electron to create an ion with 17 protons, 17 neutrons, and 18 electrons, giving it a internet negative (–one) charge. Information technology is now referred to as a chloride ion. In this example, sodium volition donate its one electron to empty its beat, and chlorine volition accept that electron to fill up its shell. Both ions now satisfy the octet rule and accept complete outermost shells. Because the number of electrons is no longer equal to the number of protons, each is now an ion and has a +1 (sodium cation) or –one (chloride anion) charge. Notation that these transactions can normally only have place simultaneously: in order for a sodium atom to lose an electron, it must exist in the presence of a suitable recipient like a chlorine cantlet.
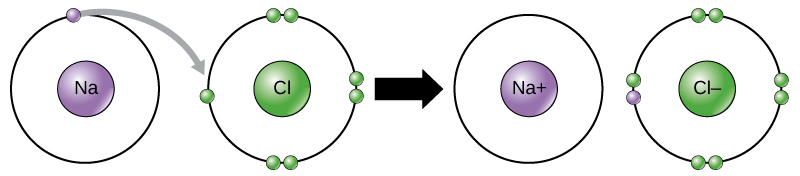
Figure ane. In the formation of an ionic chemical compound, metals lose electrons and nonmetals proceeds electrons to achieve an octet. Ionic bonds are formed between ions with contrary charges. For case, positively charged sodium ions and negatively charged chloride ions bond together to brand crystals of sodium chloride, or table salt, creating a crystalline molecule with zero internet charge.
Ionic bonds are formed between ions with opposite charges. For example, positively charged sodium ions and negatively charged chloride ions bond together to make crystals of sodium chloride, or table salt, creating a crystalline molecule with zero net charge.
Sure salts are referred to in physiology every bitelectrolytes (including sodium, potassium, and calcium), ions necessary for nerve impulse conduction, muscle contractions and water balance. Many sports drinks and dietary supplements provide these ions to replace those lost from the body via sweating during exercise.
Video Review
This video shows how ionic compounds form from anions and cations.
Covalent Bonds
Another mode the octet dominion can be satisfied is by the sharing of electrons between atoms to formcovalent bonds. These bonds are stronger and much more mutual than ionic bonds in the molecules of living organisms. Covalent bonds are commonly institute in carbon-based organic molecules, such every bit our DNA and proteins. Covalent bonds are also institute in inorganic molecules like H2O, COii, and Otwo. One, 2, or 3 pairs of electrons may exist shared, making single, double, and triple bonds, respectively. The more covalent bonds between ii atoms, the stronger their connection. Thus, triple bonds are the strongest.
The strength of different levels of covalent bonding is one of the main reasons living organisms have a difficult time in acquiring nitrogen for use in amalgam their molecules, even though molecular nitrogen, Ntwo, is the most abundant gas in the atmosphere. Molecular nitrogen consists of two nitrogen atoms triple bonded to each other and, as with all molecules, the sharing of these three pairs of electrons between the two nitrogen atoms allows for the filling of their outer electron shells, making the molecule more stable than the individual nitrogen atoms. This strong triple bond makes it difficult for living systems to intermission apart this nitrogen in gild to use it as constituents of proteins and Deoxyribonucleic acid.
The formation of water molecules provides an example of covalent bonding. The hydrogen and oxygen atoms that combine to form water molecules are bound together past covalent bonds. The electron from the hydrogen splits its time between the incomplete outer shell of the hydrogen atoms and the incomplete outer shell of the oxygen atoms. To completely fill the outer shell of oxygen, which has six electrons in its outer shell only which would exist more stable with viii, 2 electrons (one from each hydrogen cantlet) are needed: hence the well-known formula HiiO. The electrons are shared between the 2 elements to fill the outer beat of each, making both elements more than stable.
View this brusque video to see an animation of ionic and covalent bonding.
Polar and Nonpolar Covalent Bonds
There are two types of covalent bonds: polar and nonpolar. Nonpolar covalent bonds form betwixt ii atoms of the same element or between different elements that share the electrons every bit. For instance, an oxygen atom can bail with some other oxygen cantlet to fill up their outer shells. This clan is nonpolar because the electrons will be as distributed between each oxygen atom. Two covalent bonds form between the two oxygen atoms because oxygen requires 2 shared electrons to make full its outermost trounce. Nitrogen atoms will form three covalent bonds (also called triple covalent) betwixt 2 atoms of nitrogen considering each nitrogen atom needs iii electrons to fill its outermost trounce. Another case of a nonpolar covalent bond is establish in the methane (CHiv) molecule. The carbon cantlet has four electrons in its outermost shell and needs four more to fill it. Information technology gets these four from four hydrogen atoms, each atom providing one. These elements all share the electrons as, creating four nonpolar covalent bonds.
In a polar covalent bail, the electrons shared by the atoms spend more than time closer to one nucleus than to the other nucleus. Considering of the unequal distribution of electrons betwixt the different nuclei, a slightly positive (δ+) or slightly negative (δ–) charge develops. The covalent bonds between hydrogen and oxygen atoms in h2o are polar covalent bonds. The shared electrons spend more than time near the oxygen nucleus, giving it a minor negative charge, than they spend nearly the hydrogen nuclei, giving these molecules a small positive charge. Polar covalent bonds form more often when atoms that differ profoundly in size share electrons.
Examples of Covalent Bonding

Figure 2. Whether a molecule is polar or nonpolar depends both on bond type and molecular shape. Both water and carbon dioxide have polar covalent bonds, but carbon dioxide is linear, so the partial charges on the molecule abolish each other out.
Video Review
Lookout this video for another explanation of covalent bonds and how they class:
Hydrogen Bonds
Ionic and covalent bonds betwixt elements require energy to intermission. Iconic bonds are not every bit strong as covalent, which determines their behavior in biological systems. However, not all bonds are ionic or covalent bonds. Weaker bonds can too form between molecules. Two weak bonds that occur oftentimes are hydrogen bonds and van der Waals interactions. Without these two types of bonds, life as we know it would not exist. Hydrogen bonds provide many of the disquisitional, life-sustaining properties of h2o and also stabilize the structures of proteins and DNA, the building block of cells.
When polar covalent bonds containing hydrogen form, the hydrogen in that bond has a slightly positive charge because hydrogen's electron is pulled more strongly toward the other chemical element and abroad from the hydrogen. Because the hydrogen is slightly positive, it will be attracted to neighboring negative charges. When this happens, a weak interaction occurs betwixt theδ+ of the hydrogen from one molecule and the δ– charge on the more electronegative atoms of another molecule, usually oxygen or nitrogen, or within the aforementioned molecule.
Hydrogen Bonding between water molecules
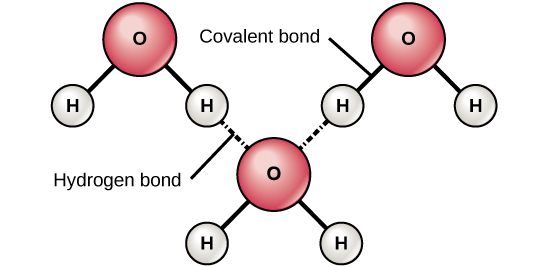
Figure three. Hydrogen bonds class between slightly positive (δ+) and slightly negative (δ–) charges of polar covalent molecules, such as water.
This interaction is called a hydrogen bond. This type of bond is common and occurs regularly between water molecules. Individual hydrogen bonds are weak and easily broken; still, they occur in very big numbers in water and in organic polymers, creating a major force in combination. Hydrogen bonds are also responsible for zipping together the DNA double helix.
Video Review
van der Waals Interactions
Similar hydrogen bonds, van der Waals interactions are weak attractions or interactions between molecules. They are also called inter-molecular forces. They occur between polar, covalently bound atoms in unlike molecules. Some of these weak attractions are acquired by temporary partial charges formed when electrons motion around a nucleus. These weak interactions between molecules are important in biological systems and occur based on physical proximity.
Radiology Technician
Figure 4. Spc. Arbor 50. LaClave practices his spinal X-ray positions utilizing Spc. Justin J. Reichelt, a radiology technician, as his mock patient to practice his skills in the health clinic at Grafenwoehr Training Surface area.
Have you or anyone you know e'er had a magnetic resonance imaging (MRI) scan, a mammogram, or an Ten-ray? These tests produce images of your soft tissues and organs (as with an MRI or mammogram) or your bones (as happens in an X-ray) by using either radiowaves or special isotopes (radiolabeled or fluorescently labeled) that are ingested or injected into the trunk. These tests provide information for affliction diagnoses by creating images of your organs or skeletal arrangement.
MRI imaging works by subjecting hydrogen nuclei, which are abundant in the water in soft tissues, to fluctuating magnetic fields, which cause them to emit their own magnetic field. This point is then read by sensors in the motorcar and interpreted by a computer to form a detailed paradigm.
Some radiography technologists and technicians specialize in computed tomography, MRI, and mammography. They produce films or images of the torso that help medical professionals examine and diagnose. Radiologists work directly with patients, explaining machinery, preparing them for exams, and ensuring that their body or torso parts are positioned correctly to produce the needed images. Physicians or radiologists then analyze the test results.
Radiography technicians can work in hospitals, doctors' offices, or specialized imaging centers. Training to become a radiography technician happens at hospitals, colleges, and universities that offering certificates, associate'south degrees, or available's degrees in radiography.
Why Life Depends on Water

Figure 5. Equally this prototype of oil and h2o shows, oil is a nonpolar compound and, hence, volition non dissolve in water. Oil and water exercise not mix.
Practise you lot always wonder why scientists spend fourth dimension looking for water on other planets? It is considering water is essential to life; even minute traces of it on another planet tin indicate that life could or did be on that planet. Water is one of the more abundant molecules in living cells and the one nearly disquisitional to life every bit we know it. Approximately 60–70 percent of your body is fabricated upwardly of water. Without it, life merely would non exist.
Water Is Polar
The hydrogen and oxygen atoms within water molecules form polar covalent bonds. The shared electrons spend more than fourth dimension associated with the oxygen atom than they do with hydrogen atoms. At that place is no overall charge to a water molecule, only in that location is a slight positive charge on each hydrogen atom and a slight negative charge on the oxygen cantlet. Because of these charges, the slightly positive hydrogen atoms repel each other and form the unique shape seen in Figure 6. Each water molecule attracts other water molecules because of the positive and negative charges in the dissimilar parts of the molecule. Water also attracts other polar molecules (such equally sugars), forming hydrogen bonds. When a substance readily forms hydrogen bonds with water, information technology can deliquesce in water and is referred to equally hydrophilic ("water-loving"). Hydrogen bonds are not readily formed with nonpolar substances similar oils and fats (Figure v). These nonpolar compounds are hydrophobic ("water-fearing") and volition not deliquesce in water.
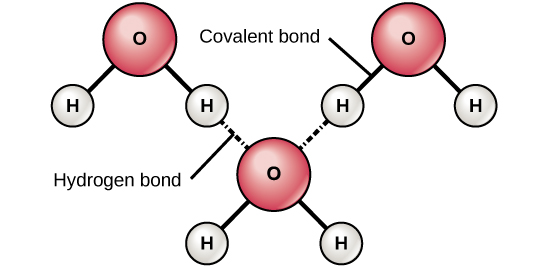
Figure 6. Hydrogen bonds form between slightly positive (δ+) and slightly negative (δ–) charges of polar covalent molecules, such as h2o.
H2o Stabilizes Temperature
The hydrogen bonds in water allow it to blot and release heat energy more than slowly than many other substances. Temperature is a measure out of the motion (kinetic energy) of molecules. As the motion increases, energy is higher and thus temperature is college. Water absorbs a cracking deal of energy before its temperature rises. Increased free energy disrupts the hydrogen bonds betwixt water molecules. Considering these bonds can be created and disrupted rapidly, water absorbs an increase in free energy and temperature changes simply minimally. This ways that water moderates temperature changes inside organisms and in their environments. As energy input continues, the residue between hydrogen-bail formation and destruction swings toward the devastation side. More than bonds are broken than are formed. This process results in the release of private water molecules at the surface of the liquid (such as a body of h2o, the leaves of a plant, or the pare of an organism) in a procedure called evaporation. Evaporation of sweat, which is 90 percent water, allows for cooling of an organism, because breaking hydrogen bonds requires an input of free energy and takes heat abroad from the trunk.
Conversely, every bit molecular movement decreases and temperatures drop, less energy is present to intermission the hydrogen bonds between water molecules. These bonds remain intact and begin to grade a rigid, lattice-similar structure (eastward.g., water ice) (Figure 7a). When frozen, water ice is less dense than liquid water (the molecules are further apart). This ways that ice floats on the surface of a trunk of water (Figure 7b). In lakes, ponds, and oceans, ice volition course on the surface of the water, creating an insulating barrier to protect the creature and plant life beneath from freezing in the h2o. If this did not happen, plants and animals living in h2o would freeze in a block of water ice and could not motility freely, making life in common cold temperatures hard or incommunicable.
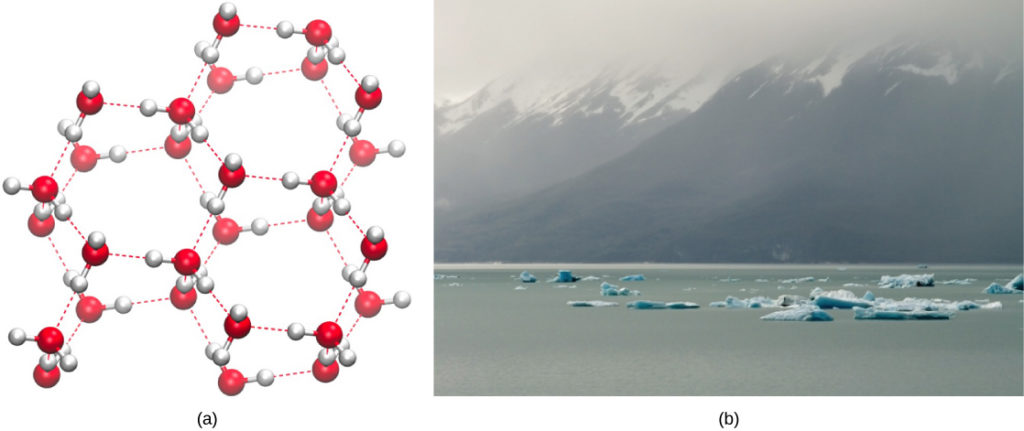
Figure 7. (a) The lattice structure of ice makes information technology less dumbo than the freely flowing molecules of liquid water. Ice'southward lower density enables it to (b) bladder on h2o. (credit a: modification of piece of work by Jane Whitney; credit b: modification of piece of work by Carlos Ponte)
Water Is an Excellent Solvent
Because water is polar, with slight positive and negative charges, ionic compounds and polar molecules can readily dissolve in information technology. Water is, therefore, what is referred to every bit a solvent—a substance capable of dissolving some other substance. The charged particles will form hydrogen bonds with a surrounding layer of water molecules. This is referred to as a sphere of hydration and serves to go along the particles separated or dispersed in the water. In the case of tabular array salt (NaCl) mixed in water (Figure 8), the sodium and chloride ions separate, or dissociate, in the water, and spheres of hydration are formed around the ions.
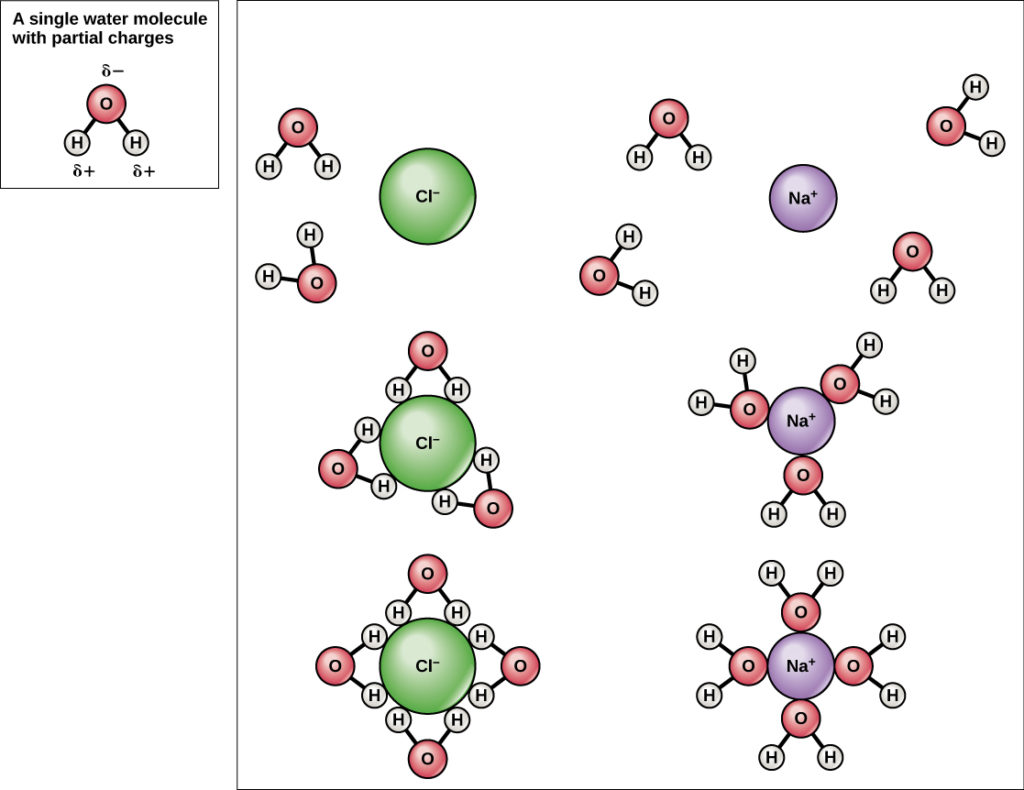
Figure viii. When table common salt (NaCl) is mixed in h2o, spheres of hydration class around the ions.
A positively charged sodium ion is surrounded past the partially negative charges of oxygen atoms in water molecules. A negatively charged chloride ion is surrounded past the partially positive charges of hydrogen atoms in water molecules. These spheres of hydration are also referred to as hydration shells. The polarity of the h2o molecule makes it an constructive solvent and is of import in its many roles in living systems.
Water Is Cohesive

Figure 9. The weight of a needle on top of water pulls the surface tension downward; at the same time, the surface tension of the water is pulling it up, suspending the needle on the surface of the water and keeping information technology from sinking. Notice the indentation in the h2o around the needle. (credit: Cory Zanker)
Have y'all ever filled upward a glass of water to the very top and so slowly added a few more than drops? Before it overflows, the water actually forms a dome-like shape above the rim of the drinking glass. This water can stay above the drinking glass because of the property of cohesion. In cohesion, water molecules are attracted to each other (because of hydrogen bonding), keeping the molecules together at the liquid-air (gas) interface, although there is no more than room in the glass. Cohesion gives rise to surface tension, the capacity of a substance to withstand rupture when placed nether tension or stress. When you drop a modest flake of paper onto a droplet of water, the paper floats on top of the water droplet, although the object is denser (heavier) than the water. This occurs because of the surface tension that is created by the water molecules. Cohesion and surface tension keep the water molecules intact and the detail floating on the top. It is even possible to "float" a steel needle on elevation of a glass of water if yous place it gently, without breaking the surface tension (Figure 9).
These cohesive forces are also related to the water'due south property of adhesion, or the attraction between water molecules and other molecules. This is observed when h2o "climbs" upwardly a straw placed in a glass of h2o. You volition notice that the water appears to be college on the sides of the straw than in the middle. This is because the h2o molecules are attracted to the straw and therefore adhere to information technology.
Cohesive and adhesive forces are important for sustaining life. For example, because of these forces, water tin flow up from the roots to the tops of plants to feed the establish.
Video Review
Practice Question
Which of the following statements is non true?
- Water is polar.
- Water stabilizes temperature.
- Water is essential for life.
- Water is the most abundant atom in Globe'south temper.
Show Respond
Statement d is non true. H2o is not the most abundant atom in Earth'due south atmosphere—nitrogen is.
Check Your Understanding
Answer the question(due south) below to run across how well you sympathize the topics covered in the previous section. This short quiz doesnot count toward your course in the class, and you tin retake it an unlimited number of times.
Use this quiz to check your understanding and decide whether to (ane) study the previous department farther or (2) movement on to the next department.
forestandindeford.blogspot.com
Source: https://courses.lumenlearning.com/wmopen-nmbiology1/chapter/atomic-bonds/
0 Response to "when an atom bonds to another atom what is it trying to do"
Post a Comment